Oncotarget published "Safety and initial efficacy of ablative radioembolization for the treatment of unresectable intrahepatic cholangiocarcinoma" which reported that to investigate safety, response, and survival after ablative glass microsphere 90Y radioembolization for unresectable intrahepatic cholangiocarcinoma. A retrospective review of 37 radioembolizations in 28 patients treated with a single compartment dose of 190 Gy encompassing >75% of the largest tumor was performed.
Objective response at 3 months was 94.1%. Median OS was not reached and the 30-month OS rate was 59%, with a median follow-up of 13.4 months. FFP in the radiated field and overall FFP at 30 months were 67% and 40%, respectively. Radioembolization of unresectable intrahepatic cholangiocarcinoma with ablative intent has a high response rate, promising survival, and is well tolerated.
Radioembolization of unresectable intrahepatic cholangiocarcinoma with ablative intent has a high response rate, promising survival, and is well tolerated.
Dr. Ricardo Paz-Fumagalli from The Mayo Clinic said, "Intrahepatic cholangiocarcinoma (iCCA) is the second most common primary hepatic malignancy following hepatocellular carcinoma (HCC)"
Surgery is the gold standard treatment for localized iCCA, but few patients are candidates for resection at presentation and many tumors recur locally after treatment. Cytotoxic chemotherapy has demonstrated only a modest survival benefit, although targeted molecular and immunotherapies show potential for improved outcomes with reduced toxicity.
Transarterial radioembolization using Yttrium-90-containing microspheres for the treatment of HCC has advanced over the past two decades from a palliative intent treatment to an ablative modality applicable as first line definitive therapy in select patients. Administering high doses of radiation to expendable volumes of liver, also known as radiation segmentectomy and lobectomy, has improved both the safety and efficacy of radioembolization. Whether a similar dose relationship is present with cholangiocarcinoma remains unknown. Additionally, unresectable iCCA also presents with blood supply variation and anatomic complexity, which may affect outcomes.
This study aimed to evaluate the initial safety and efficacy of ablative radioembolization for the treatment of unresectable iCCA, in which >75% of the tumor was treated with >190 Gy Medical Internal Radiation Dose.
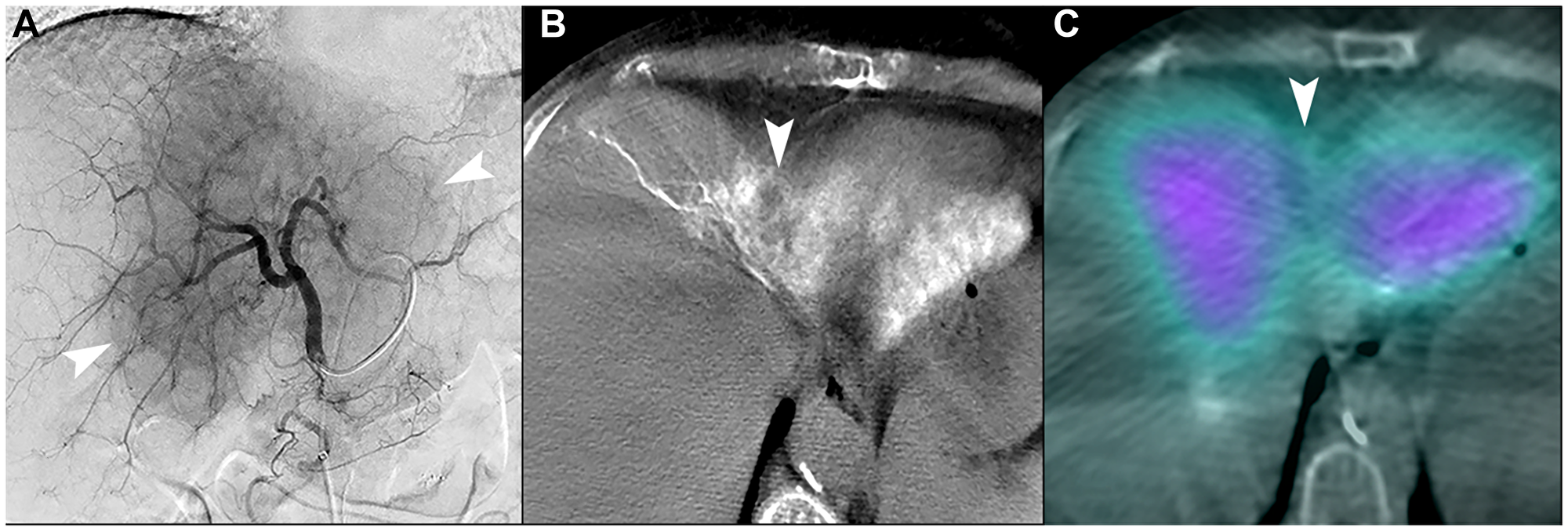
Figure 7: Unfavorable macrovascular and microvascular arterial conduit in two patients with intrahepatic cholangiocarcinoma. (A) Single image from proper hepatic arteriogram shows a large hypervascular iCCA (arrowheads). Cone-beam computed tomography of multiple branches (not shown) demonstrated that tumor blood supply would require treatment of non-expendable liver, which could not be adequately attenuated with distal angiosomal truncation representing unfavorable macrovascular conduit. Ablative intent radioembolization was not offered. (B) Cone-beam computed tomography of a different patient shows an iCCA in segment IVA with poor enhancement of tumor (arrowhead). (C) Bremsstrahlung SPECT/CT after radioembolization showed poor uptake of microspheres in the tumor (arrowhead), representing an unfavorable microvascular conduit.
The Paz-Fumagalli Research Team concluded in their Oncotarget Research Output that the limitations of the present study include the small sample size and retrospective nature. This study only included patients with iCCA and excluded other biliary tract cancers with potentially worse prognoses. Only patients who were candidates for ablative radioembolization were included.
Patients who received doses greater than 190 Gy were included, but no comparison was made with patients with similar disease that received lower doses. An intention-to-treat analysis was not performed, as this was outside the scope of the exploratory design of this study. This study did not adequately evaluate whether radioembolization can be employed as a sole therapy in patients with unresectable tumors. Response rates were measured using mRECIST which has not been extensively studied for iCCA. An analysis of cross-sectional imaging features and the impact on response was not performed. Compartment and voxel dosimetry analyses were not performed because complete data was not available. Lastly, the concept of vascular conduit quality, as applied clinically by the interventional radiologists that participated in this study, has not been externally validated.
Sign up for free Altmetric alerts about this article
DOI - https://doi.org/10.18632/oncotarget.28060
Full text - https://www.oncotarget.com/article/28060/text/
Correspondence to - Ricardo Paz-Fumagalli - paz.ricardo@mayo.edu
Keywords - Yttrium-90, radioembolization, cholangiocarcinoma, angiography, radiation dosimetry
About Oncotarget
Oncotarget is a biweekly, peer-reviewed, open access biomedical journal covering research on all aspects of oncology.
To learn more about Oncotarget, please visit https://www.oncotarget.com or connect with:
SoundCloud - https://soundcloud.com/oncotarget
Facebook - https://www.facebook.com/Oncotarget/
Twitter - https://twitter.com/oncotarget
LinkedIn - https://www.linkedin.com/company/oncotarget
Pinterest - https://www.pinterest.com/oncotarget/
Reddit - https://www.reddit.com/user/Oncotarget/
Oncotarget is published by Impact Journals, LLC please visit https://www.ImpactJournals.com or connect with @ImpactJrnls
Media Contact
MEDIA@IMPACTJOURNALS.COM
18009220957x105




