The cover for issue 2 of Oncotarget features Figure 4, "Combination therapy TA99/ICB reduced the lung tumor burden in the B16 model of metastases," published in "Improved therapeutic efficacy of unmodified anti-tumor antibodies by immune checkpoint blockade and kinase targeted therapy in mouse models of melanoma" by Pérez-Lorenzo, et al. which reported that here, the authors showed that removing immune suppression and enhancing stimulatory signals increased the anti-tumor activity of unmodified TA99 antibodies with a significant reduction of growth of solid tumors and lung metastases in mouse models of melanoma.
Immune checkpoint blockade enhanced the efficacy of TA99, which was associated with greater CD8 /Foxp3 , NK1.1 and dendritic cell infiltrates, suggestive of an increased anti-tumor innate and adaptive immune responses.
Moreover, they found an improved therapeutic effect when YUMM tumor-bearing mice were treated with TA99 combined with MEKi and immune checkpoint blockade.
The Oncotarget findings suggest that MEKi induced an increased expression of tumor-associated antigens, which in combination with anti-tumor antibodies, generated a robust adaptive anti-tumor response that was sustained by immune checkpoint inhibition therapy.
The Oncotarget findings suggest that MEKi induced an increased expression of tumor-associated antigens, which in combination with anti-tumor antibodies, generated a robust adaptive anti-tumor response that was sustained by immune checkpoint inhibition therapy.
The authors postulate that combining anti-tumor antibodies with standard-of-care strategies such as immune checkpoint blockade or targeted therapy, will improve therapeutic outcomes in cancer.
Dr. Angela M. Christiano from The Columbia University said, "It is well accepted that tumor development and progression is usually controlled by immunosurveillance mechanisms in which specific and non-specific immunological responses are constantly mounted against tumor cells."
Passive administration of anti-tumor antibodies generally functions by targeting malignant cells through IgG-mediated antibody-dependent cellular cytotoxicity, which is a rapid but relatively short-acting anti-tumor response.
Alternatively, they and others also demonstrated that the administration of anti-tumor antibodies induces long-lasting FcR-dependent tumor specific immunity in the host, with kinetics consistent with an induced adaptive immune response against the tumor.
In this model, anti-tumor antibodies, alone or in combination with chemotherapy, will promote innate cell-mediated ADCC, and the capture and processing of antigens by antigen presenting cells, with the subsequent stimulation and homing of antigen-specific effector T lymphocytes to the tumor site, leading to tumor elimination, a phenomenon the researchers and others referred to as the “vaccinal effect”.
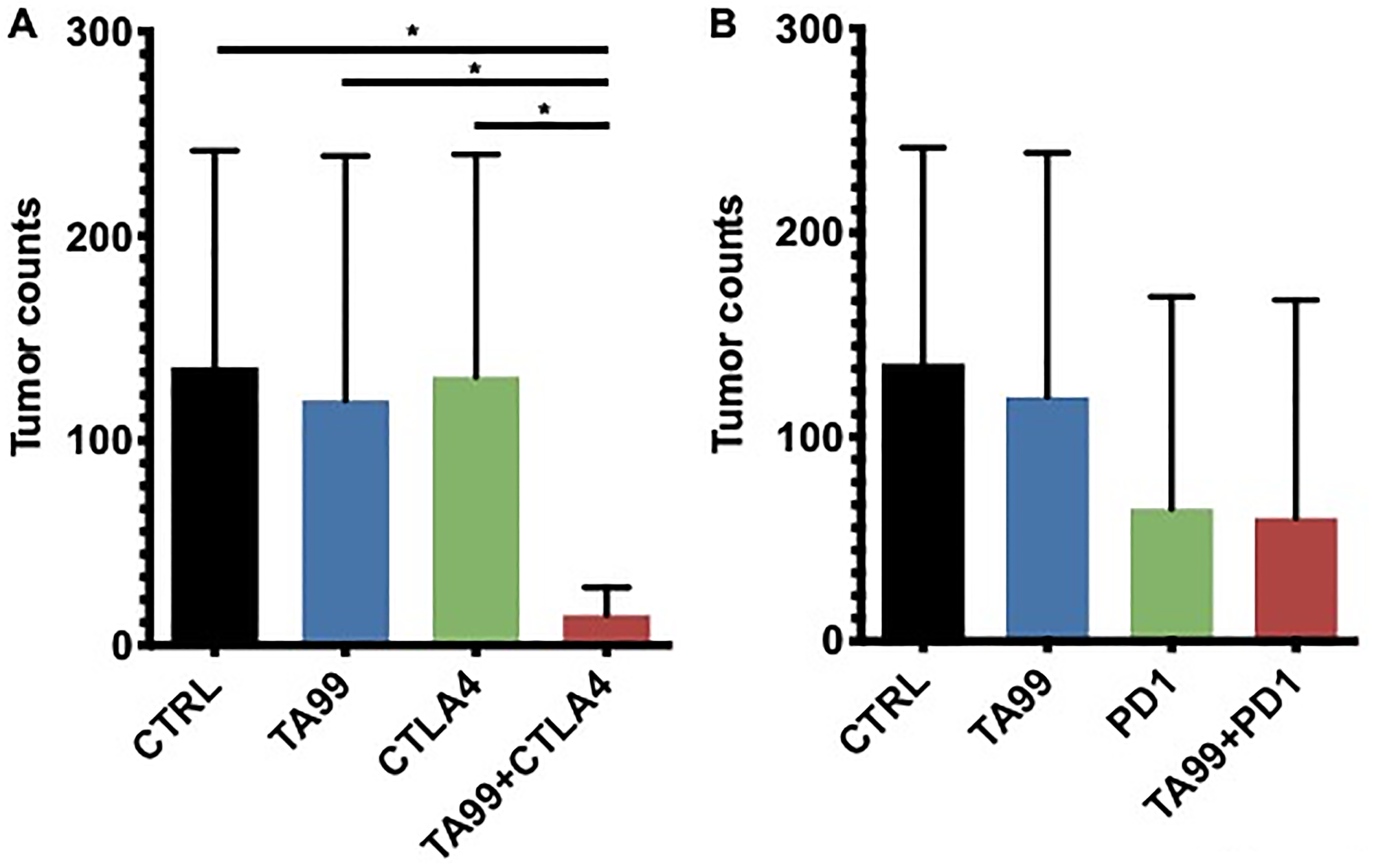
Figure 4: Combination therapy TA99/ICB reduced the lung tumor burden in the B16 model of metastases. C57BL/6 mice were inoculated B16 cells via tail vein injection, and treatment with anti-CTLA4 (A) or anti-PD1 (B) alone or in combination with TA99 was administered as described in Figure 3A. After 21 days, lungs were harvested and fixed, and metastatic nodules counted. The combination of TA99 mAb with immune checkpoint blockade reduced the lung metastases burden. Mean ± SEM is shown. *p < 0.05.
With the use of the B16 and YUMM mouse models of melanoma and the anti-TYRP1 mouse monoclonal antibody TA99, we demonstrated that the therapeutic effects of these unmodified anti-tumor antibodies can be enhanced by ICB through the stimulation of both innate and adaptive anti-tumor immune responses.
In addition, they found that the MEK inhibitor -induced increased expression of melanosomal antigens further enhanced the anti-melanoma response to combination therapy with anti-tumor antibodies and immune checkpoint blockade in mouse models of melanoma.
The Christiano Research Team concluded in their Oncotarget Research Paper, "Together with our preclinical data, these results invite further clinical investigation of unmodified anti-tumor antibodies in combination with ICB and targeted therapies, and may represent promising and innovative therapeutic interventions for the successful management of patients with advanced melanoma and other cancers."
Sign up for free Altmetric alerts about this article
DOI - https://doi.org/10.18632/oncotarget.27868
Full text - https://www.oncotarget.com/article/27868/text/
Correspondence to - Angela M. Christiano - amc65@cumc.columbia.edu
Keywords - anti-tumor antibodies, targeted therapy, immunotherapy, combination therapies, melanoma
About Oncotarget
Oncotarget is a biweekly, peer-reviewed, open access biomedical journal covering research on all aspects of oncology.
To learn more about Oncotarget, please visit https://www.oncotarget.com or connect with:
SoundCloud - https://soundcloud.com/oncotarget
Facebook - https://www.facebook.com/Oncotarget/
Twitter - https://twitter.com/oncotarget
LinkedIn - https://www.linkedin.com/company/oncotarget
Pinterest - https://www.pinterest.com/oncotarget/
Reddit - https://www.reddit.com/user/Oncotarget/
Oncotarget is published by Impact Journals, LLC please visit http://www.ImpactJournals.com or connect with @ImpactJrnls
Media Contact
MEDIA@IMPACTJOURNALS.COM
18009220957x105




