Oncotarget recently published "Ewing sarcoma family of tumors-derived small extracellular vesicle proteomics identify potential clinical biomarkers" which reported that outside of tumor biopsy, no clinically relevant ESFT biomarkers exist.
Additionally, tumor burden assessment at diagnosis, monitoring of disease responsiveness to therapy, and detection of disease recurrence are limited to radiographic imaging.
To identify new, clinically relevant biomarkers the authors evaluated the proteome of a subset of ESFT-derived small extracellular vesicles.
They performed the first high-quality proteomic study of ESFT-derived sEVs from 5 ESFT cell lines representing the most common EWS-ETS fusion types and identified 619 proteins composing the core ESFT sEV proteome.
They compared these core proteins to databases of common plasma-based proteins and sEV-associated proteins found within the healthy plasma to identify proteins unique or enriched within ESFT. From these analyses, two membrane-bound proteins with biomarker potential were selected, CD99/MIC2 and NGFR, to develop a liquid-based assay enriching of ESFT-associated sEVs and detection of sEV mRNA cargo.
They employed this immuno-enrichment approach to the diagnosis of ESFT utilizing plasma from both localized and metastatic ESFT pediatric patients and cancer-free controls and showed significant diagnostic power.
In this study, the Oncotarget authors demonstrate utilization of circulating ESFT-associated sEVs in pediatric patients as a source of minimally invasive diagnostic and potentially prognostic biomarkers.
The Oncotarget authors demonstrate utilization of circulating ESFT-associated sEVs in pediatric patients as a source of minimally invasive diagnostic and potentially prognostic biomarkers
Dr. Andrew K. Godwin from The University of Kansas Cancer Center said, "Ewing Sarcoma Family of Tumors (ESFT) encompass a group of highly aggressive pediatric osseous and soft tissue malignancies thought to originate from primordial bone marrow-derived mesenchymal stem cells and consists of small round blue cells with minimal stroma and differentiation."
Outside of the presence of overt metastatic disease, no clinically relevant predictive biomarkers exist which are indicative of the increased risk of recurrence in localized ESFT patients.
Various other clinical prognostic factors such as age, tumor location, tumor size, and histologic response to induction therapy; however, have not been incorporated within pediatric ESFT treatment risk stratification.
Standard circulating tumor markers are not applicable to ESFT. Serum lactate dehydrogenase has been most consistently associated with aggressive disease but lacks ESFT specificity.
Traditionally, ESFT was considered a diagnosis of exclusion, but over the past few decades, with the introduction of immunohistochemical markers, e.g., CD99/MIC2 and detection of the oncogenic chimeric fusion involving the Ewing sarcoma RNA binding protein 1 gene, which is a hallmark of ESFT, the accuracy of diagnosis has considerably improved.
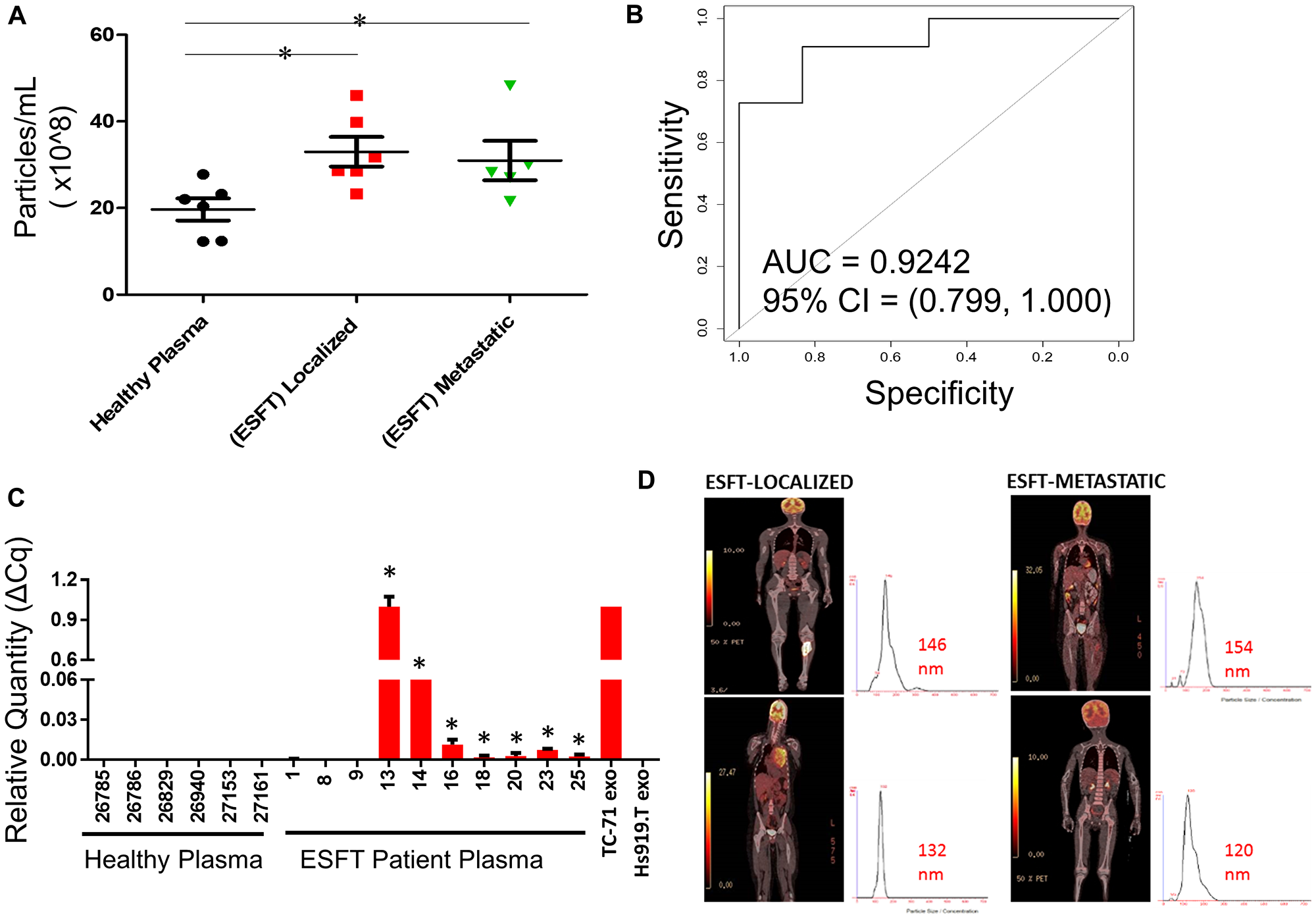
Figure 6: Enrichment of ESFT-sEVs from clinical ESFT plasma samples. (A) Total concentration of sEV's isolated from 250 μL of pediatric ESFT (n = 10) and healthy plasma (n = 6). (B) Area Under the Curve (AUC) analysis (A) of total sEV counts in cases and controls following immuno-enrichment. (C) Detection of the EWS-ETS transcript by qRT-PCR, * denotes samples that crossed threshold and are considered positive, total of 7/10. cDNA from sEVs derived from TC-71 and Hs919.T cell lines served as positive and negative controls, respectively. (D) Localized and metastatic pediatric ESFT patient isolated sEVs demonstrating size threshold between 120–154 nm.
The essential role of EWS-ETS fusion transcripts makes it less likely to be down-regulated or non-existent during tumor progression, convincingly supporting their routine utility for diagnostic assessment in ESFT tumor biopsied tissues but also in circulating tumor-derived sEVs.
In this study, the Oncotarget authors report for the first time the proteome of ESFT-derived sEVs/exosomes and develop a clinically useful test based on immuno-enrichment of ESFT-sEVs and detection of the EWS-ETS fusion transcripts.
The Godwin Research Team concluded in their Oncotarget Research Paper that in order to further advance the development of sEVs as biomarkers in ESFT, their ongoing studies are integrating protein markers identified through our study into our prototypic microfluidic chip.
As discussed above, they have recently demonstrated the quantitative measurement of EWS-FLI1 mRNA copy numbers in pPNET-derived sEVs.
Although a rare disease, the development of this type of integrated assay could aid in the diagnosis of all members of the ESFT family.
The researchers are currently in process of developing a single microfluidic platform using our validated capture reagents to streamline the enrichment of tumor-derived sEVs and quantitative measurement of EWS-ETS fusion transcripts in ESFT. Studies of ESFT tumor-derived sEVs may reveal possible new important therapeutic targets, as well as perhaps yield RNAs prognostic for tumor aggressiveness and chemotherapeutic sensitivity allowing clinicians to better treat this pediatric malignancy.
DOI - https://doi.org/10.18632/oncotarget.27678
Full text - https://www.oncotarget.com/article/27678/text/
Correspondence to - Andrew K. Godwin - agodwin@kumc.edu
Keywords - Ewing sarcoma, EWS-ETS, biomarkers, extracellular vesicles, exosomes
About Oncotarget
Oncotarget is a biweekly, peer-reviewed, open access biomedical journal covering research on all aspects of oncology.
To learn more about Oncotarget, please visit https://www.oncotarget.com or connect with:
SoundCloud - https://soundcloud.com/oncotarget
Facebook - https://www.facebook.com/Oncotarget/
Twitter - https://twitter.com/oncotarget
LinkedIn - https://www.linkedin.com/company/oncotarget
Pinterest - https://www.pinterest.com/oncotarget/
Reddit - https://www.reddit.com/user/Oncotarget/
Oncotarget is published by Impact Journals, LLC please visit http://www.ImpactJournals.com or connect with @ImpactJrnls
Media Contact
MEDIA@IMPACTJOURNALS.COM
18009220957x105




