Oncotarget published "JunD accentuates arecoline-induced disruption of tight junctions and promotes epithelial-to-mesenchymal transition by association with NEAT1 lncRNA" which reported that the effects of low and high concentrations of arecoline on the stability of tight junctions and EMT induction were studied.
A microarray analysis confirmed involvement of a MAPK component, JunD, in regulating tight junction-associated genes, specifically ZO-1. Results established that although arecoline-induced phosphorylation of JunD downregulated expression of ZO-1, JunD itself was modulated by the lncRNA-NEAT1 in presence of arecoline.
Increased NEAT1 in tissues of HNSCC patients significantly correlated with poor disease prognosis.
Here the authors show that the NEAT1-JunD complex interacted with the ZO-1 promoter in the nuclear compartment, downregulated the expression of ZO-1 and destabilized the tight junction assembly.
Consequently, silencing NEAT1 in arecoline-exposed cells not only downregulated the expression of JunD and stabilized expression of ZO-1, but also reduced expression of the EMT markers, Slug and Snail, indicating its direct regulatory role in arecoline-mediated TJ disruption and disease progression.
Dr. Urmi Chatterji from The University of Calcutta said, "In the past few years, there has been a drastic rise in the incidence of head and neck cancers worldwide."
Dr. Urmi Chatterji from The University of Calcutta said, "In the past few years, there has been a drastic rise in the incidence of head and neck cancers worldwide."
The International Agency for Research on Cancer has declared the psychoactive areca nut to be carcinogenic to humans and chewing betel nut increases the risk of oropharyngeal cancer, independent of use of tobacco and alcohol.
Multiple signaling pathways, such as MAPK/JNK and PI3K/AKT signaling pathways, are involved in the pathogenesis of HNSCC, and are indispensable for the growth and survival of cancer cells.
Cellular homeostasis and subsequent signaling are in turn regulated by cell adhesion molecules, which are severely disrupted in cancers.
JunD homodimers activate rat HSCs which contribute to the fibrogenic process through TIMP-1 activation.
Here they report that JunD leads to down regulation of ZO-1 and abrogates tight junctions via activation of the JunD-NEAT1 axis in betel nut chewing HNSCC patients of India.
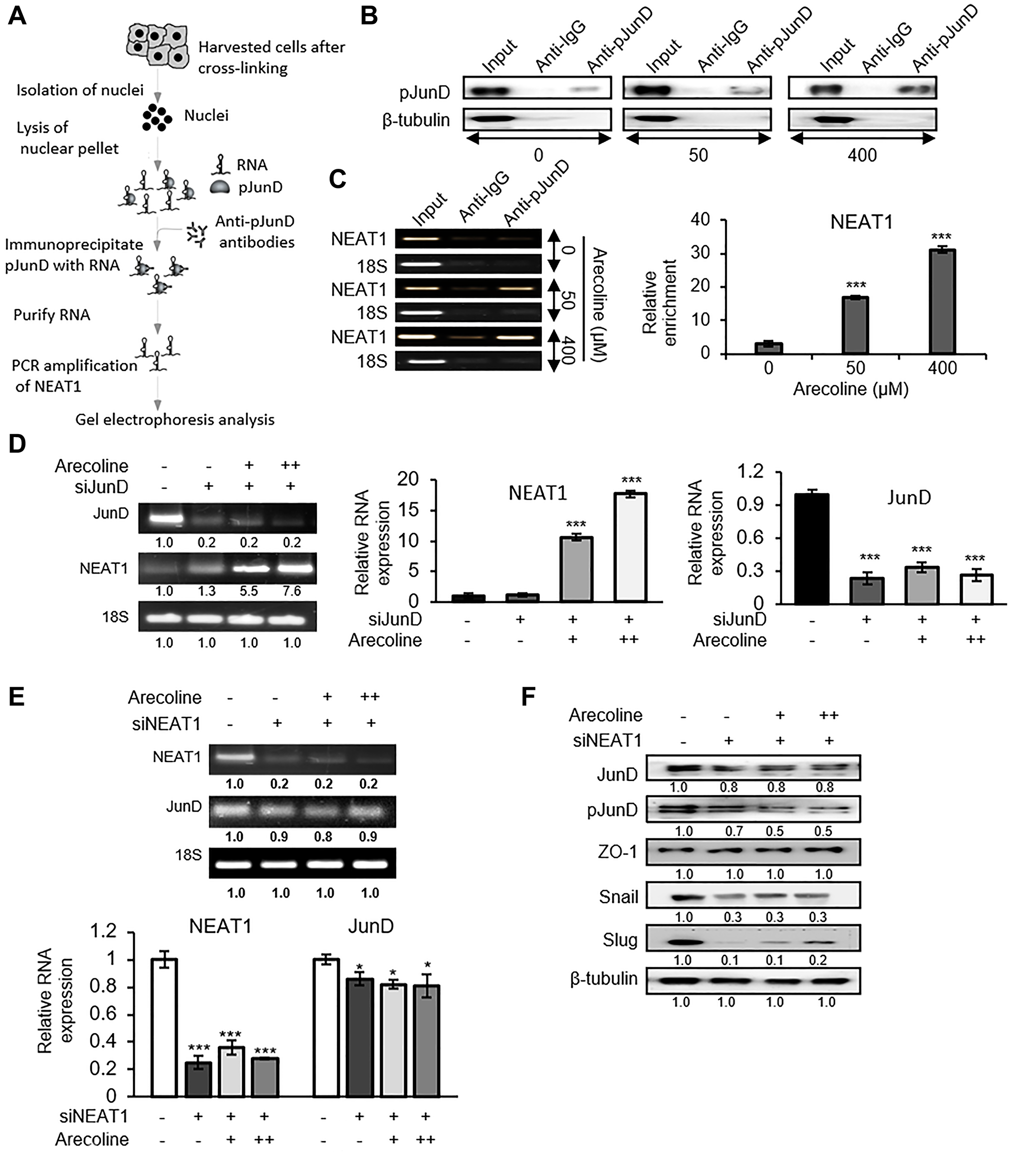
Figure 8: NEAT1 plays a pivotal role in JunD-mediated downregulation of ZO-1. (A) Schematic representation of RNA immunoprecipitation (RIP) performed to determine the interaction between pJunD and NEAT1. (B) Expression of pJunD in absence and presence of 50 μM and 400 μM arecoline after immunoprecipitation with pJunD specific antibody. RIP with anti-IgG, indicating non-specific antibody binding, served as the negative control. (C) Expression of NEAT1 following RIP by semi-qPCR and RT-PCR. 18S served as control for non-specific amplification. (D) Expression of JunD and NEAT1 after silencing JunD in HEp-2 cells followed by treatment without and with 50 μM (+) and 100 μM (++) arecoline. The relative RNA expressions are represented for NEAT1 and JunD. (E) Expression of JunD and NEAT1 after silencing NEAT1 in HEp-2 cells followed by treatment without and with 50 μM (+) and 100 μM (++) arecoline. (F) Differential protein expression of tight junction and EMT markers in HEp-2 cells after silencing NEAT1 followed by treatment without and with 50 μM (+) and 100 μM (++) arecoline. β-tubulin was used as an internal control for all western blots. All the experiments were performed three times. Each value is the mean ± S.D. of three different replicate experiments, each performed in triplicate. *p < 0.1 and ***p < 0.001.
The Chatterji Research Team concluded in their Oncotarget Research Output that in accordance, it was imperative to evaluate whether NEAT1 coordinated with JunD in response to arecoline and modulated ZO-1 expression.
Since binding of JunD to the ZO-1 promoter has been previously demonstrated, it may be conjectured that this binding is in all probability enhanced in the presence of NEAT1 lncRNA, since individually NEAT1 and JunD are enriched in the nuclear fraction in response to arecoline treatment.
The results substantiated the specific association of NEAT1 lncRNA with JunD in the nuclear fraction in the presence of arecoline, confirming the alternative mechanism by which arecoline modulated JunD in HEp-2 cells.
Although silencing JunD did not reduce the expression of NEAT1 in presence or absence of arecoline, silencing NEAT1 suppressed the expression of JunD, emphasizing the importance of NEAT1 association with JunD to render it functional and bind to the ZO-1 promoter.
Therefore, in cancer cells exposed to arecoline, JunD is activated not by phosphorylation alone but by interaction with NEAT1 to suppress the expression of ZO-1 and destabilize structural integrity of TJs.
DOI - https://doi.org/10.18632/oncotarget.28026
Full text - https://www.oncotarget.com/article/28026/text/
Correspondence to - Urmi Chatterji - urmichatterji@gmail.com
Keywords - arecoline, tight junction, head and neck cancer, lncRNA-NEAT1, JunD
About Oncotarget
Oncotarget is a bi-weekly, peer-reviewed, open access biomedical journal covering research on all aspects of oncology.
To learn more about Oncotarget, please visit https://www.oncotarget.com or connect with:
SoundCloud - https://soundcloud.com/oncotarget
Facebook - https://www.facebook.com/Oncotarget/
Twitter - https://twitter.com/oncotarget
LinkedIn - https://www.linkedin.com/company/oncotarget
Pinterest - https://www.pinterest.com/oncotarget/
Reddit - https://www.reddit.com/user/Oncotarget/
Oncotarget is published by Impact Journals, LLC please visit https://www.ImpactJournals.com or connect with @ImpactJrnls
Media Contact
MEDIA@IMPACTJOURNALS.COM
18009220957x105




